Answer:
The fertilizer has 0.3kg of nitrogen, 0.1kg of phosphorus and 0.8kg of potassium.
Step-by-step explanation:
The series 12-4-32 indicates the percentage of each element in the fertilizer. So, in this case, there are 12% of nitrogen, 4% of phosphorus and 32% of potassium, and the rest will be oxygen.
knowing that the 100% of the fertilizer is equivalent to 2.5kg, we can calculate the mass of each element using a mathematical rule of three:
• Mass of Nitrogen:
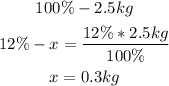
• Mass of Phosphorus:
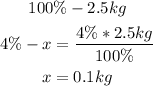
• Mass of potassium:
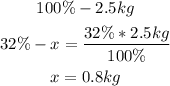
So, the fertilizer has 0.3kg of nitrogen, 0.1kg of phosphorus and 0.8kg of potassium.