We know that
• Pure acid is added to a 5% acid solution.
,
• To obtain 95 liters of 28% solution.
Based on the given information, we can define the following formula
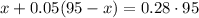
Where 0.05 is 5% and 0.28 is 28%.
Solving for x, we have
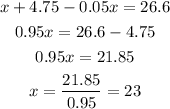
This means there will be 23 liters of pure acid to 72 liters of 5% solution. (23 + 72 = 95 liters in total).
Now, to find the liters of 100% pure acid, we use the following equation.
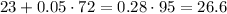
Therefore, 26.6 liters of acid is needed.