ok. We are given the following equation:
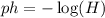
Where:
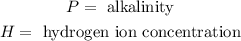
Since we are asked to determine "H" we will solve for "H" in the equation. To do that we will first multiply both sides by -1:
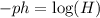
Now, we will use the following property of logarithms:
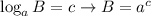
Applying the property and having into account that:
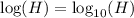
We get:

Now we substitute the given value of "pH = 4.5":
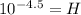
Solving the operation:
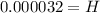
Therefore, the hydrogen ion concentration of the fluid is 0.000032