Answer:
The final volume of the gas is 0.81L.
Step-by-step explanation:
1st) It is necessary to write all the variables given before start, and make sure that volume is in L, the temperature in K, and the pressure in atm, to use the ideal gas equation properly:
Variables at noon:
• V1: 730mL (0.73L)
• T1: 37 °C (310.15K)
• P1: 1atm
Variables at evening:
• V2: this is the volume that we have to calculate.
• T2: 26°C (299.15K)
• P2: 660mmHg (0.87atm)
2nd) Now we can replace the values in the formula and calculate the final volume:
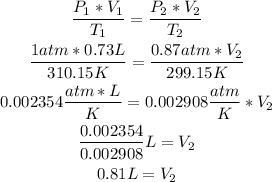
So, the final volume of the gas is 0.81L.