The equation that gives the heat for a change in temperature is:
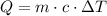
Where Q is the heat, m is the mass, c is the specific heat capacity and ΔT is the change in temperature.
So, we can calculate Q by substituting the given values:
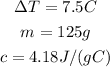
So, we have:

So, the heat needed is 3918.75 J or approximately 3900 J.