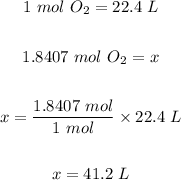Answer
41.2 L
Step-by-step explanation
Given that:
The mass of oxygen gas (O₂) = 58.9 grams
What to find:
The volume of 58.9 grams of oxygen gas (O₂) at STP.
Step-by-step solution:
The first step is to convert 58.9 grams of oxygen gas (O₂) given to moles using the mole formula:
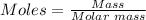
The molar mass of oxygen gas (O₂) is 31.998 g/mol.
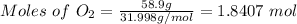
The final step is to convert the 1.8407 moles of oxygen gas (O₂) to volume using the conversion factor below.
Conversion factor: The molar volume of any gas at STP is 22.4 L. That is, one mole of any gas has a volume of 22.4 L or 22,400 mL.
So,