ANSWER
The usual oxidation state of lithium is +1
Step-by-step explanation
Lithium is a group 1 metal with an atomic number of 3. Its electronic configuration of lithium atom is written below as
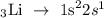
In the above configuration, lithium has one electron in its outermost shell. Hence, the valence of electron is 1
During chemical reaction, lithium will lose an electron to attain it octet structure and it will attain the electronic configuration of Helium
Since it will be losing one electron, therefore it usual oxidation number is +1u