Given data
*The specific heat of zinc is c = 386 J/kg
*The given mass of zinc is m = 0.25 kg
*The initial temperature of zinc is t = 0°C
*The final temperature of zinc is T = 28°C
The formula for the amount of heat energy required to raise the temperature of 0.25 kilogram of zinc from 0°C to 28°C is given as
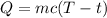
Substitute the values in the above expression as
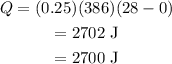
Thus, the amount of heat energy required to raise the temperature of 0.25 kilogram of zinc from 0°C to 28°C is Q = 2700 J and the correct answer is (b)