First, find the number of moles it must contain.
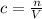
Where n is the number of moles, c = 0.5 and V = 600 mL. But, we need to use the volume in Liters, so we divide 600mL by 1000.
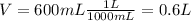
Once we have the correct unit, find n.
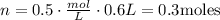
Now, we need to know what volume would contain 0.3 moles, using the same formula.
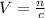
Where n = 0.3 moles and c = 1.2 mol/L.
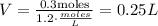
At last, transform 0.25 Liters into mL.
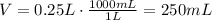
Therefore, the volume is 250 mL.