Hello
To solve this problem, we need to write out the chemical formula of Nickel (ii) Nitrate and know it's molecular mass
The chemical formula of the compound is given as
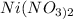
The molecular mass is given as 182.703g/mol
With this information above, we can say that
6 moles of oxygen atoms is present in 182.703g of Ni(NO₃)₂
x moles of oxygen would be present in 2.6g of Ni(NO₃)₂
We can equate both sides and solve for x
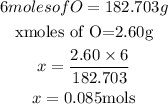
From the calculation above, we can see that 2.60mols of nickel (ii) nitrate will contain 0.085moles of Ni(NO₃)₂
Note: The founding basis for this calculation is known as the mole-mass relationship