the Given the initial ratio of the sulfuric acid to water
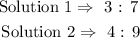
So to obtain the ratio value of the sulfuric in solution 1, multiply each water ratio by 3/7
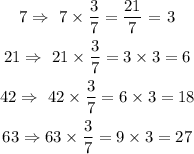
So to obtain the ratio value of the sulfuric in solution 2, multiply each water ratio by 4/9
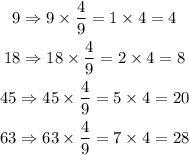
In conclusion, you will notice that when solution 1 has a water ratio of 63, the acidic content is 27, while in solution 2 when water ratio is 63 the acidic content is 28.
Hence, Solution 2 is more acidic