We are given the mass and volume of an object. We can calculate the density using the following formula:-
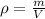
Where:
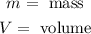
Now, we plug in the values:
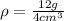
Solving the operation:
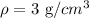
Therefore, the density of the material is 3 g/cm^3. Therefore, the material with the density that is the closest to the one we calculated is aluminum.