Answer: The reaction given can be classified as a neutralization reaction, and the chemical equation provided is not balanced.
The balanced chemical equation can be written as:
![3Ca(OH)_2+2H_3PO_4\operatorname{\rightarrow}Ca_3(PO_4)_2+6H_2O]()
Step-by-step explanation:
The question requires us to classify the reaction given and determine if it is balanced or not.
The chemical equation presented by the question was:
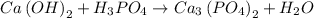
The chemical equation given presents the reaction between a base (Ca(OH)2) and an acid (H3PO4) to form salt (Ca3(PO4)2) and water (H2O), which can be classified as a neutralization reaction.
To determine if the chemical equation is balanced, we need to check if the number of atoms of each element is the same on both sides:
- Ca: there is 1 Ca atom on the left side and 3 on the right side;
- O: there are 2+4 = 6 O atoms on the left side and 2*4 + 1 = 9 O atoms on the right side;
- H: there are 2 + 3 = 5 H atoms on the left side and 2 H atoms on the right side;
P: there is 1 P atom on the left side and 2 P atoms on the right side.
Therefore, the chemical equation presented is not balanced.
To balance this chemical equation, we can follow the order: metals > non-metals > hydrogen > oxygen.
Let's start with Ca: we can adjust the coefficient of Ca(OH)2 from 1 to 3 to achieve 3 Ca atoms on both sides:
![3Ca(OH)_2+H_3PO_4\operatorname{\rightarrow}Ca_3(PO_4)_2+H_2O]()
Next, let's adjust the amount of P atoms: we can adjust the coefficient of H3PO4 from 1 to 2 to have 2 P atoms on both sides:
![3Ca(OH)_2+2H_3PO_4\operatorname{\rightarrow}Ca_3(PO_4)_2+H_2O]()
At this point, we have 12 H atoms on the left side (3*2 + 2*3) and 2 H atoms on the right side: we can adjust the coefficient of H2O from 1 to 6 to have the same amount of H atoms on both sides:
Now, we need to check the amount of O atoms: at this point, there are 3*2 + 2*4 = 14 O atoms on the left side and 2*4 + 6 = 14 O atoms on the right side, thus we don't need to adjust any more coefficients.
The balanced chemical equation can be written as: