Answer:
103 mL.
Step-by-step explanation:
What is given?
Volumen 1 (V1) = 257.9 mL.
Pressure 1 (P1) = 0.309 atm.
Pressure 2 (P2) = 0.771 atm.
What do we need? Volume 2 (V2).
Step-by-step solution:
We can identify that this is a Boyle's law problem: Boyle's law states that the volume of a given mass of gas varies inversely with the pressure when the temperature is kept constant. An inverse relationship is described in this way. As one variable increases in value, the other variable decreases. The formula of this law is the following:
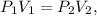
where P is pressure and V is volume. We want to find the final volume, which would be 'v2', so let's solve for this unknown value and replace the given data, like this:
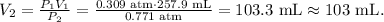
The answer would be that the final volume is 103 mL. As pressure increase, the volume decrease.