Answer: 5.95 grams
Calculations:
We are given :
• mass of water = ?
• ΔT, =( Tfinal - T initial ) = ( 40 -30 )= 10 °C
,
• Q ,= 250J
,
• Specific heat capacity(,c,) = 4.2J/g•°C
We will the formula :
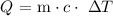
Therefore ;
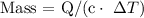
Replacing the given parameters, we get that mass =
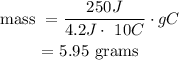
• This means that the mass required = 5.95 grams.