The given concentration, 0.40% (m/v), is a mass volume percent concentration. It indicates us that there are 0.40g of NaCl in every 100mL of solution.
We can use this information to detemine how many grams of NaCl are there in 3.0L (3000mL) of solution:
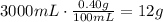
Now, convert this amount of mass to moles using NaCl molar mass:
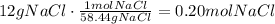
It means that the corect answer is 0.20 moles of NaCl.