Answer
Step-by-step explanation
Given:
2Al + 3Cl₂ 2AlCl₃
Molar mass of AlCl3 => 133 g/mol
Moles of Cl₂ = 25.0 mol
What to find:
The grams of aluminum chloride that can be produced.
Step-by-step solution:
Step 1: Determine the mole of AlCl₃ produced.
From the given chemical equation for the reaction,
3 mol Cl₂ produce 2 mol AlCl₃
So 25.0 mol Cl₂ will produce
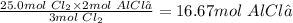
16.67 moles of AlCl₃ produced.
Step 2: Calculated the grams of AlCl₃ produced.
Using the mole formula
![undefined]()