Answer:
5.00 L.
Step-by-step explanation:
What is given?
Volume 1 (V1) = 4.37 L.
Temperature 1 (T1) = 37.12 °C + 273 = 310.12 K.
Temperature 2(T2) = 81.93 !°C + 273 = 354.93 K.
What do we need? Volume 2 (V2).
Step-by-step solution:
This is a Charles's Law problem. Charles's Law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. The absolute temperature is temperature measured with the Kelvin scale.
The formula that we're going to use based on this law is:
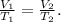
Where V is volume and T is temperature.
We want to find volume 2 (V2), so let's solve for 'V2' and replace the given data that we have in the new formula, like this:
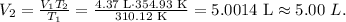
The new volume is 5.00 L.