Step 1
The chemical formula of iron (III) sulfate is the next one:
Fe2(SO4)3
As we can see, there are 3 x 1 atom of S, 3 atoms of S
----------------
Step 2
Information needed:
The atomic masses of:
Fe) 55.8 g/mol
S) 32.0 g/mol
O) 16.0 g/mol
(Please, the periodic table is useful here)
----------------
Step 3
The % of S in Fe2(SO4)3 is calculated as follows:
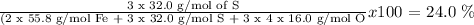
Answer: 24 % of S