The first step we have to follow is to convert the given mass of sodium hydrogen carbonate to moles using its molecular mass (MM=84kg/kmol):
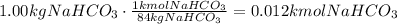
Now, use the stoichiometric ratio given by the equation to find how many moles are needed to produce 0.012kmoles of NaHCO₃:
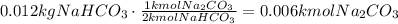
Now, convert the estimated amount of Na2CO3 to mass using its molecular mass (MM=106kg/kmol):
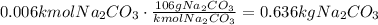
Convert the mass in kg to g:
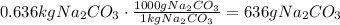
It means that the answer is 636g of soda ash are needed to produce 1.00 kg of sodium hydrogen carbonate.