Answer
243.69 K
Step-by-step explanation
Given:
Initial temperature, T₁ = 37 °C = (37 + 273.15 K) = 310.15 K
Initial pressure, P₁ = 5.6 atm
Final pressure, P₂ = 4.4 atm
What to find:
The temperature to which the gases must be raised in order for the can to explode.
Step-by-step solution:
According to Amonton's Law, it states that the pressure of an ideal gas varies directly with the absolute temperature when the volume of the sample is held constant.
The final temperature of the gas can be calculated using Amonton's law formula given by
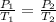
Putting the values of the given parameters into the equation:

The temperature to which the gases must be raised in order for the can to explode is 243.69 K