Answer:
15.46atm
Explanations:
According to the ideal gas equation;

where:
• P is the ,pressure ,of the gas
,
• V is the volume of the gas = 1.1L
,
• n is the number of moles = 0.7mol
,
• R is the ,Gas constant, = 0.08206Latm/molK
,
• T is the temperature = 23 + 273 = 296K
Substitute the given parameters into the formula
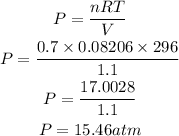
Hence the pressure of the gas that will be exerted is 15.46atm