ANSWER

Step-by-step explanation
Parameters given:
Linear expansivity of aluminum, α = 24 * 10^(-6) C^(-1)
Initial temperature,m T1 = 33°C
Initial volume of liquid, V1 = 285 mL
(a) To find the amount of liquid that the cup can hold on a summer day, apply the formula for volume expansivity:
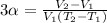
where T2 = temperature on a cold day
V2 = volume on a cold day
Therefore, solving for V2, we have that the volume that the cup can hold on a cold day will be:
![\begin{gathered} 3*24*10^^(-6)=(V_2-285)/(285(-4-33)) \\ \\ 0.000072=(V_2-285)/(285*37)=(V_2-285)/(-10545) \\ \\ V_2-285=0.000072*-10545=0.75924 \\ \\ V_2=285+0.75924 \\ \\ V_2=285.76\text{ }mL \end{gathered}]()
That is the answer.
(b) To find the volume that the cup can hold on a spring day, apply the same formula but for T2 = 17°C:

That is the answer.