Answer:
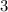
Step-by-step explanation:
Here, we want to know the number of moles of water molecules present in the hydrate
Firstly, we need to get the percentage by mass of water
To get this, we have to divide the percentage lost to heating by the total mass and convert to a percentage
Mathematically, we have that as:
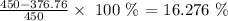
What this means is that the percentage by the molar mass of water is still 16.276%
To get the number of moles of water, let us call it n
The molar mass of water divided by the total mass of the hydrate as a percentage is equal to 16.276%
Thus, we have it that;
The molar mass of n moles of water is (18 * n = 18n g/mol : 1 mole of water has a mass of 18g)
For the anhydrous part, the molar mass is 278 g/mol
Thus, we have the percentage as:
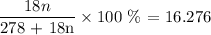
From the above, we can get the value of n
Thus, we have that as:

The number of hydrate molecule is 3