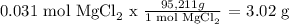Answer
Mg(OH)2 is the limiting reactant
Mass of MgCl2 produced = 3.02 g
Step-by-step explanation
Given: The balanced chemical equation
mass of Mg(OH)2 = 1.85 g
mass of HCl = 3.71
Solution
Mg(OH)2
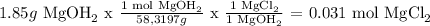
HCl
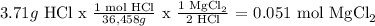
Therefore Mg(OH)2 is the limiting reactant.
Calculation of MgCl2 mass