We know that the half life of this element is 41.3 days.
We have to find how much will remain of a sample of 2 grams after 86 days.
The half life of 41.3 days means that the mass after 41.3 days will become half of what it was.
We can express this as:
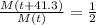
As this is represented with an exponential model like this:
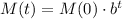
we can use the half-life to find the parameter b:
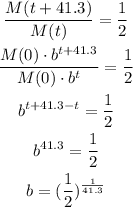
Then, knowing that the initial mass M(0) is 2 grams, we can express the final model as:
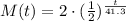
We then can calculate the mass after t = 86 days as:
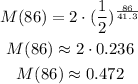
Answer: the mass after 86 days will be 0.472 grams.