ANSWER
The amount of valence electron in an atom is equivalent to the group the atom is located
Step-by-step explanation
Valency is define as the combining power of an element. Valence electrons are electrons located at the outer most shell of an element.
Valence electrons tell us the group a particular element belongs to and also the chemical properties of the element.
For example, sodium atom
Sodium has the below electronic configuration
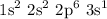
The structure above represents a sodium atom. The structure contains a single electron at the outer most shell of the atom. The single electron is called the valence electron of sodium. Hence, sodium belongs to group 1
Therefore, the amount of valence electron in an atom is equivalent to the group the atom is located