Answer: first option (or letter A): 20.3 g LizN
Step-by-step explanation:
The question requires us to calculate the mass of Li3N produced when 1.75 moles of Li and excess of N2 are used in the following chemical reaction:
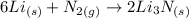
To solve this problem, we must consider the stoichiometry of the reaction to calculate the amount of moles of Li3N produced and then use the molar mass of Li3N (34.83 g/mol) to convert the number of moles in mass of Li3N.
According to the chemical equation, 2 moles of Li3N are produced when 6 moles of Li are reacting. Thus, we can write:
6 mol Li ---------------------- 2 mol Li3N
1.75 mol Li ------------------ x
Solving for x, we'll have:
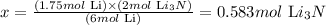
Therefore, 0.583 moles of Li3N are obtained from 1.75 moles of Li.
Now, considering the molar mass of Li3N (34.83 g/mol):
1 mol Li3N --------------- 34.83g Li3N
0.583 mol Li3N ------- y
Solving for y, we'll have that 0.583 moles of Li3N correspond to 20.3 g of this compound.
The best option to answer this question is the first one (or letter A): 20.3 g LizN.