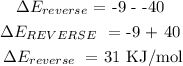ANSWER
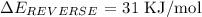
Step-by-step explanation
Given information
The energy on the product side = -40 KJ
The energy on the reactant side = -9kJ
Assume the reaction is a reversible reaction

For a reverse reaction, the product becomes the reactant and the reactant is the new product formed
Step 1: Write the general formula for calculating a change in energy
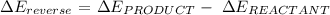
Step 2: Substitute the given data into the formula in step 1