Kinetics - Rate of a reaction - Reaction order
A first order reaction is a reaction in which the rate is proportional to the concentration of only one reactant.
Answer:
We have the following reaction and reaction rate:
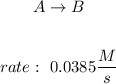
The rate of the reaction is expresed as:
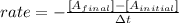
In this case:
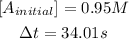
So now we calculate the final concentration of A:
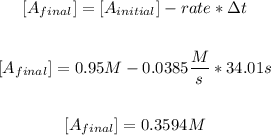
So the final concentration of A is 0.3594M.