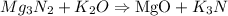
Step 1) We must balance our equation.
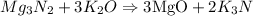
(we will work with this equation)
---------------------------------------------------------------------------------------------------------
Step 2) The limiting reactant = K2O
We need to find the mass of K3N, so we need its molar mass (use the periodic table):
Molar Mass K3N = 130 g/mol (approx.)
---------------------------------------------------------------------------------------------------------
Step 3) Use the stoichiometry
3 x 1 mole of K2O ----------- 2 x 130 g of K3N
14 moles of K2O----------- X
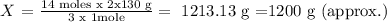
Answer: Mass of K3N = 1200 g (approx.)