The first step is to convert the volume of solution in mililiters to liters, use a conversion factor to do it:
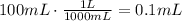
Now, multiply the volume of the solution by its concentration to find the number of moles that are needed to make that solution (remember that M=mol/L):
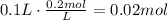
Finally, convert this amount of moles to grams using the molecular weight of K2CrO4 (MW=194.2g/mol):
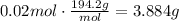
The amount of grams of K2CrO4 to make 100ml of solution is 3.884g.