We know that carbon will react with zinc oxide to produce zinc and carbon dioxide. So, first we must write the balanced equation:
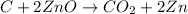
Now, knowing that 135 grams of ZnO reacted and using that:
- 1 mol of ZnO: 81.41 grams of ZnO
- 1 mol of CO2: 2 mol of ZnO
- 1 mol of CO2: 44.01 grams of CO2
Writing it as a table,
Answer:
36.5 g of CO2 will be produced