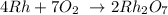Answer:
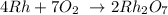
Step-by-step explanation:
Here, we want to balance the given chemical equation
We can start by balancing the oxygen atoms
We have 7 on the left and 2 on the right
To make a balance, we place 7 before the molecular oxygen on the left and 2 on the given oxide
That means we would be having 4 Rh on the left
To balance the Rh, we have to place 4 on the left side
Thus, we have the balanced equation as: