Let x = volume of 20% solution=0.2
Let y = volume of 30% solution=0.3
Equation of total volume:
x + y = 90
Equation of amount of acid:
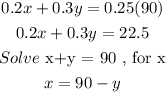
Substitute into the second equation
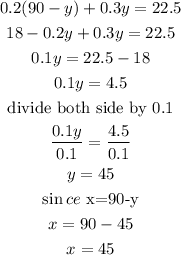
Therefore 45 liters of 20% solution and 45 liters of 30% solution
Hence the volume of 20% acid = 45 litres
the volume of 30% acid = 45 litres