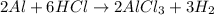Answer:
Based on the given option, the unbalanced form is Option A
![Al+HCl\operatorname{\rightarrow}AlCl_3+H_2]()
Explanations:
Equation is known to be balanced when the number of moles of element at the reactant is equal to that of product.
Aluminum = Al
Hydrochloric acid = HCl
The reaction formed by Aluminum and hydrochloric acid is expressed as:
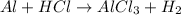
The balanced form of the reaction will be expressed as: