Let:
• A ,be the number of millilitres (mL) of solution A used.
,
• B ,be the number of mL of solution B used.
We know that Dale uses four times as much solution A as solution B, meaning
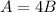
Now, we know that we will end up with 570 mL of pure alcohol in the final solution. Using the dilution of both A and B (20% means 0.2 and 15% is 0.15) we would have that:
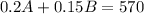
We would have the following system of equations:
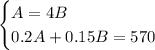
Substituting equation 1 in equation 2 and solving for B :
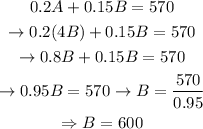
Substituting in equation 1 and solving for A:
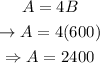
This way, we can conclude that 2400 mL of solution A and 600mL of solution B were used.