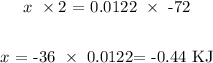Answer:
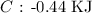
Step-by-step explanation:
Here, we want to get the amount of heat released in KJ
From the change in enthalpy given and the equation of reaction, we know that 2 moles of HBr would lead to that amount of heat
Now, let us get the actual amount of heat released
We need to get the actual number of moles of HBr produced
Mathematically, we can calculate that by dividing the mass of HBr by its molar mass
We have that as:
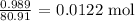
From the reaction information:
-72 KJ was released by 2 moles
x KJ would be released by 0.0122 mol
To get the value of x, we have it that: