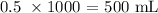Answer:
Step-by-step explanation:
Here, we want to get the maximum volume of solution that can be produced
Given:
Molarity = 1.8 M
Number of Moles = 0.9 moles
Find:
Volume
Equation Used:
Number of moles = molarity * volume
Answer:
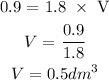
To convert this to mL, we multiply the volume by 1000 since 1 L = 1000 mL
Thus, we have it that: