There are 2.04*10^-4 moles of hydrogen.
To start the calculation it is necessary to use the molar mass of glycine:
- Glycine molar mass: 75.08 g/mol.
From the formula of glycine, we know that in 75.08g of glycine, there are 5 moles of hydrogen. So, in 3.06*10^-3g of glycine will be another amount of hydrogen, and we can calculate it with a mthematical Rule of Three:
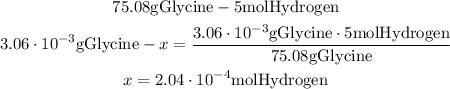
So, there are 2.04*10^-4 moles of hydrogen in 3.06*10^-3g of glycine.