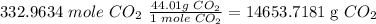Answer
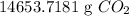
Procedure
Considering the following balanced equation.
2CH₃OH + 3O₂ ---> 2CO₂ + 4H₂O
To determine the grams of CO₂ that will be produced, you will first need to get the grams of water produced, then convert the grams of water into moles of water and use the molar proportios to get the moles of CO₂. Lastly, convert the moles of CO₂ into grams.
Additional data:
Dnsity of water = 1 g H₂O=/ of H₂O
CO₂ molar mass = 44.01 g/mol
H₂O molar mass = 18.02 g/mol
Calculations
Convert to mass
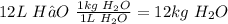
Convert to moles
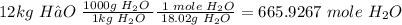
Convert to moles of CO₂ using the relationship
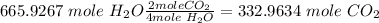
Convert to grams of CO₂