Hello!
When we round something to the nearest tenth, it means that the number must have just one decimal place.
Let's analyze this number and round it:
7050.387
How can we write 387 as one number?
Well, it's very close to 400, and we can "hide" these zeros.
Let's try it:
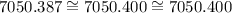
Answer:
7050.4.