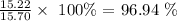Answer:
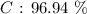
Step-by-step explanation:
Here, we want to get the percentage by mass of calcium carbonate in the limestone
We start by writing an equation of chemical reaction
The reaction involves calcium carbonate reacting with hydrochloric acid to give calcium chloride, carbon dioxide and water
We have the equation as follows:
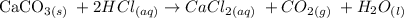
Now, let us work with the extra information
16.89 g of calcium chloride are produced
Let us get the number of moles of calcium chloride produced
We can get that by dividing the mass by the molar mass of calcium chloride
The molar mass of calcium chloride is 111 g/mol
So, the number of moles will be:

From the equation,
1 mole of calcite gave 1 mole of calcium chloride, then 0.1522 mole of calcium chloride was produced by 0.1522 mole of calcite
To get the mass of calcite that produced this, we have to multipply the number of moles by the molar mass of calcite
The molar mass of calcite is 100 g/mol
That means the mass of calcite that produced the calcium chloride will be:
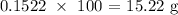
What this mean is that 15.22 g of calcite produced the given mass of calcium chloride
The percentage by mass of calcite in the limestone will be: