Use the radioactive decay formula using t_0 as the half life:
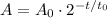
Substitute the initial amount of radioactive element A_0=100g, the half life t_0=10y and the time period t=20y to find the remaining amount after 20 years:
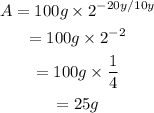
Therefore, after 20 years, there are 25 grams left.