Answer
B. When ΔH is negative and ΔS is positive
Step-by-step explanation
The spontaneity of a reaction can be determined by the sign of the Gibbs free energy change. For a spontaneous reaction, the change in Gibbs free energy should be negative, that is:
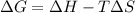
The spontaneity of a process can depend on the temperature. Since the temperature value here corresponds to the absolute temperature, this implies that T > 0 for any T. Therefore, to have a negative difference for any temperature value, the change in enthalpy (ΔH), should be negative and the change in entropy (ΔS) should be positive so that we always subtract a positive number from a negative number. This corresponds to a negative value in ΔG.
Hence, the