The percent composition are the percent in mass of each atom in the molecule.
Since we know that the molar mass of the substance is 334.0 g/mol, we can use it to figure out how much of each atom we have per molecule.
Starting from carbon, we know it is 75.42% of the mass.
In 1 mol of the substance, there are 334.0 g, 75.42% of which is from carbon atoms.
So, in 1 mol of the substance, there are the following mass of carbon:
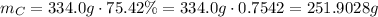
The aromic mass of the carbon atom is 12.0107 g/mol.
That is, in 1 mol of the substance -> 251.9028 g of C.
That mass of carbon is equivalent to:
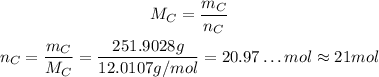
So, in 1 mol of the substance -> 251.9028 g of C -> 21 mol of C. This means that for each molecule of the substance we have 21 atoms of carbon.
Now, we can just repeat the process for Hydrogen, Nitrogen and Oxygen.
Hydrogen has an atomic mass of 1.00794 g/mol, and the percent composition of it is 6.63%, so:
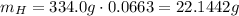
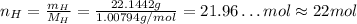
So, for each molecule of the substance there are 22 atoms of hydrogen.
Nitrogen has an atomic mass of 14.0067 g/mol, and the percent composition of it is 8.38%, so:
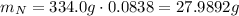
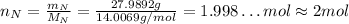
So, for each molecule of the substance there are 2 atoms of nitrogen.
Oxygen has an atomic mass of 15.9994 g/mol, and the percent composition of it is 9.57%, so:
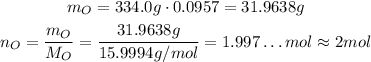
So, for each molecule of the substance there are 2 atoms of oxygen.
To summarize, we have, for each molecule of the substance:
- 21 carbon
- 22 hydrogen
- 2 nitrogen
- 2 oxygen
So, the molecular form of the substance is C₂₁H₂₂N₂O₂.