The balanced equation of the reaction is:
CoCl2 + F2 → CoF2 + Cl2
Now, we will follow the next steps to solve the question.
1. We find the moles present in 124.13 g of cobalt(II)fluoride (CoF2) using the molar molar mass of CoF2. The molar mass of CoF2 is: 96.93g/mol
2. By stoichiometry we find the moles of fluorine (F2) needed. Since the ratio CoF2 to F2 is 1, the moles will be the same as those produced from cobalt(II)fluoride.
3. We find the grams of fluorine by multiplying the moles by the molar mass of fluorine. The molar mass of fluorine is 38.00 g/mol
Let's proceed with the calculations:
1. Moles of CoF2
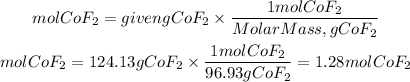
2. Moles of F2
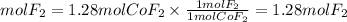
3. Grams of F2
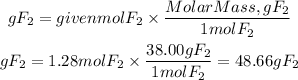
Answer: To produce 124.13 grams of cobalt(II)fluoride are required 48.66grams of fluorine