Answer:
288 g.
Step-by-step explanation:
Measurements => Density.
Density is the ratio of the mass of an object to its volume:
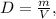
where D is density, m is mass, and V is volume.
We have two data: the volume (32.1 mL) and the density (8.96 g/mL) and we need to find the mass.
We can solve for 'm' in the given equation and replace the given data, as follows:
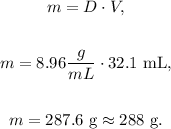
The answer would be that the mass is 288 g.