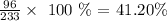Answer:
Iron 24.03%
Carbon 30.90%
Hydrogen 3.86%
Oxygen 41.20 %
Step-by-step explanation:
Here, we want to get the percentage composition of the given compound
What this means is that we want to get the percentage composition of each of the elements present in the compound
Firstly, we calculate the molar mass of the given compound
We can calculate the molar mass by adding the atomic masses of the elements and their multiplicities
Mathematically, we have that as:
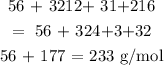
Now, let us get the percentage compositions
For Iron, we have:
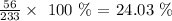
For Carbon, we have:
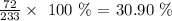
For Hydrogen, we have:
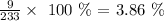
For Oxygen, we have: