ANSWER
8 moles of water
Step-by-step explanation
The major products formed when an organic compound undergo combustion reaction are water and carbon dioxide
Below is the combustion formula

In the question above, 4 moles of methane reacts with oxygen
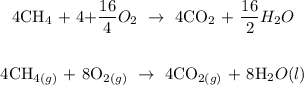
Therefore, 4 moles of methane will give 8 moles of water